Starting a Clinical Trial in Georgia Pharma/Medical Device /Advanced Therapies
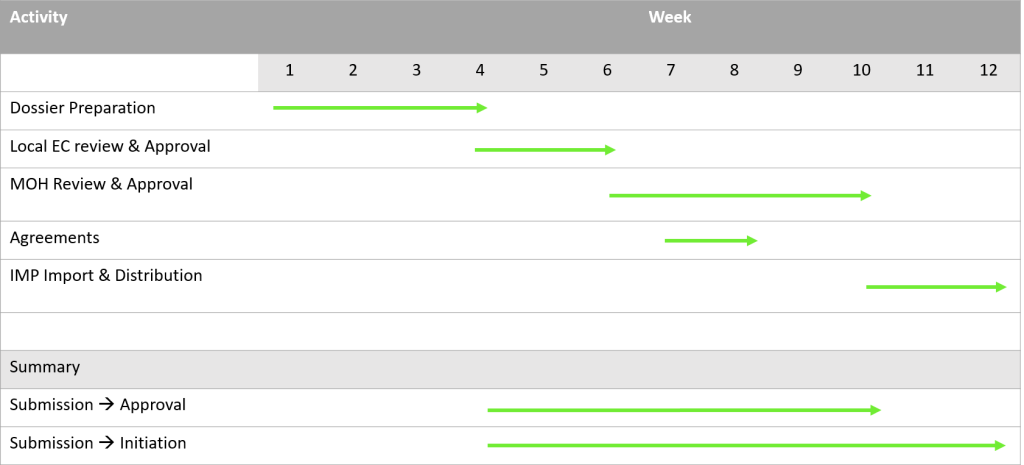
First step is to obtain an approval from the Local Ethical Committee (LEC) from each of the study sites. Arranging the submission dossier usually takes 3-5 weeks and the review process in the LECs takes an additional 1-2 weeks. The LECs approval is needed for submitting the dossier to the Georgian Ministry of Health (MoH), which is the next step. The review of your study in the MoH will take about 3 weeks.
Agreements with study sites and investigators is done in parallel to the regulatory approval process. We can assist you with the agreement negotiations. It usually takes us 2-3 weeks to achieve that.
Last step is to obtain an import permit. This will take another 2 weeks.
So, summing it all, within 3 months from “green light” you will have your study ready to go.